- Home
- About drugs
- VERZENIO FILM-COATED TABLET 100MG [SIN15789P]
VERZENIO FILM-COATED TABLET 100MG [SIN15789P]
Active ingredients: Abemaciclib
Product Info
VERZENIO FILM-COATED TABLET 100MG
[SIN15789P]
Product information
Active Ingredient and Strength | ABEMACICLIB 100 MG |
Dosage Form | TABLET, FILM COATED |
Manufacturer and Country | LILLY DEL CARIBE, INC.PUERTO RICO |
Registration Number | SIN15789P |
Licence Holder | DKSH SINGAPORE PTE. LTD. |
Forensic Classification | PRESCRIPTION ONLY MEDICINES |
Anatomical Therapeutic Chemical (ATC) code | L01EF03 |
1 INDICATIONS AND USAGE
1.1 Early Breast Cancer
Verzenio (abemaciclib) in combination with endocrine therapy is indicated for the adjuvant treatment of adult patients with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative, node-positive early breast cancer at high risk of recurrence (see section 14.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
1.2 Advanced or Metastatic Breast Cancer
Verzenio (abemaciclib) is indicated:
in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer.
in combination with fulvestrant for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Schedule
When used in combination with fulvestrant, tamoxifen or an aromatase inhibitor, the recommended dose of Verzenio is 150mg taken orally twice daily. Refer to the Full Prescribing Information for the recommended dose of fulvestrant, or tamoxifen or aromatase inhibitor being used.
Pre/perimenopausal women treated with the combination of Verzenio plus endocrine therapy should be treated with a gonadotropin-releasing hormone agonist according to current clinical practice standards.
For early breast cancer, Verzenio should be taken continuously for two years or until disease recurrence, or unacceptable toxicity.
For advanced or metastatic breast cancer, continue treatment until disease progression or unacceptable toxicity.
Verzenio may be taken with or without food [see Clinical Pharmacology (12.3) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information].
Instruct patients to take their doses of Verzenio at approximately the same times every day.
If the patient vomits or misses a dose of Verzenio, instruct the patient to take the next dose at its scheduled time. Instruct patients to swallow Verzenio tablets whole and not to chew, crush, or split tablets before swallowing. Instruct patients not to ingest Verzenio tablets if broken, cracked, or otherwise not intact.
2.2 Dose Modification
Dose Modifications for Adverse Reactions
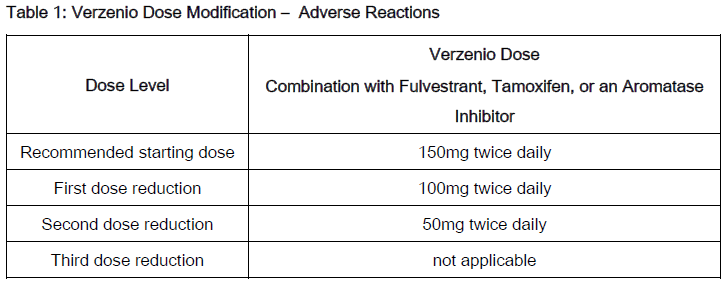
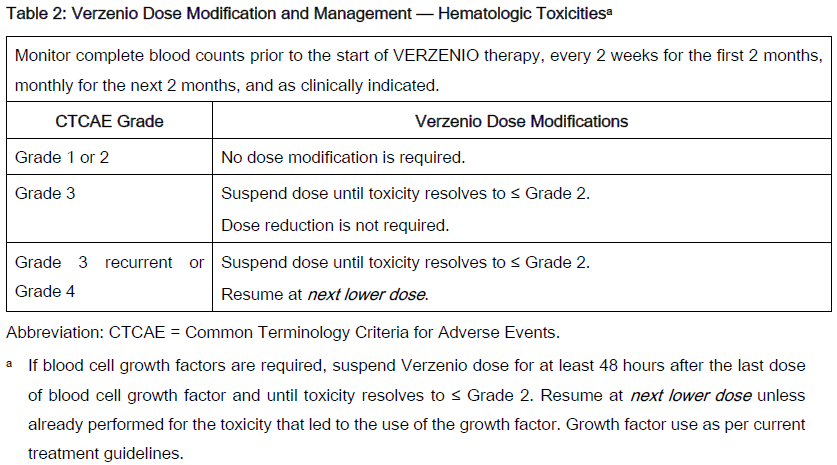
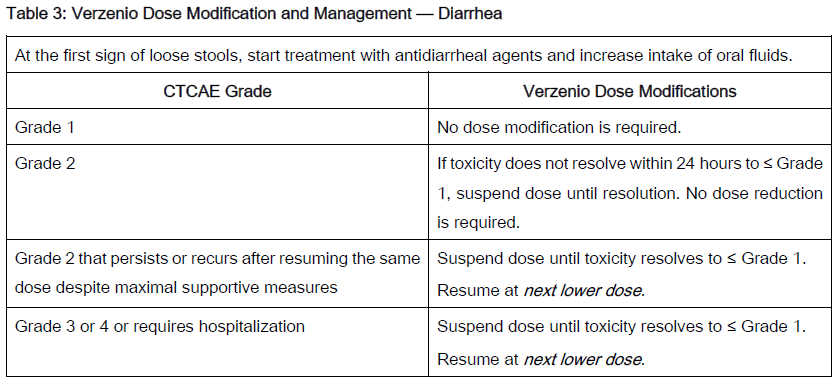
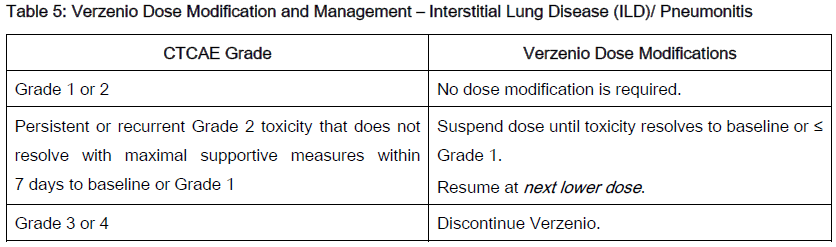
The recommended Verzenio dose modifications for adverse reactions are provided in Tables 1–7. Discontinue Verzenio for patients unable to tolerate 50mg twice daily.

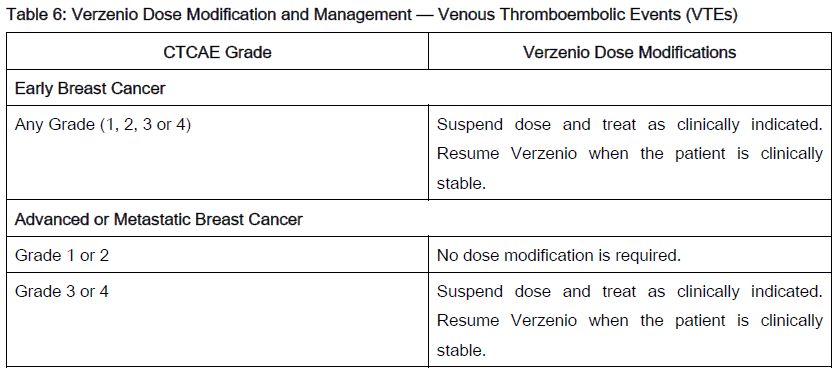
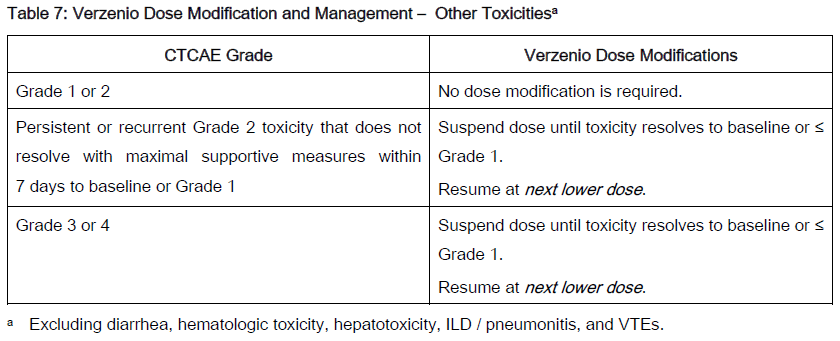
Refer to the Full Prescribing Information for coadministered fulvestrant, tamoxifen or aromatase inhibitor for dose modifications and other relevant safety information.
Dose Modification for Use with Strong and Moderate CYP3A Inhibitors
Avoid concomitant use of the strong CYP3A inhibitor ketoconazole.
With concomitant use of strong CYP3A inhibitors other than ketoconazole, in patients with recommended starting doses of 150mg twice daily, reduce the Verzenio dose to 100mg twice daily. In patients who have had a dose reduction to 100mg twice daily due to adverse reactions, further reduce the Verzenio dose to 50mg twice daily. If a patient taking Verzenio discontinues a strong CYP3A inhibitor, increase the Verzenio dose (after 3–5 half-lives of the inhibitor) to the dose that was used before starting the strong inhibitor [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information].
With concomitant use of moderate CYP3A inhibitors, monitor for adverse reactions and consider reducing the Verzenio dose in 50 mg decrements as demonstrated in Table 1, if necessary.
Dose Modification for Patients with Severe Hepatic Impairment
For patients with severe hepatic impairment (Child Pugh-C), reduce the Verzenio dosing frequency to once daily [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information].
Refer to the Full Prescribing Information for coadministered fulvestrant, tamoxifen or aromatase inhibitor for dose modification requirements for severe hepatic impairment.
4 CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients listed in Section 11, Description – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
